Rosnilimab, a selective depleter of pathogenic T cells, is a potential game changer for the treatment of moderate-to-severe rheumatoid arthritis (RA).
In a new Phase 2b clinical trial, rosnilimab demonstrated rapid symptomatic improvement driving patients into low disease activity (LDA) across diverse patient populations, including difficult-to-treat patients, by three months that continued to improve through six months. The data further indicated these responses were durable for at least three additional months off-drug. Rosnilimab also demonstrated favorable safety and tolerability, particularly when compared to the safety profiles of standard of care biologics or JAK inhibitors.1
“Witnessing rosnilimab, with its novel mode of action, dramatically reduce RA disease activity through six months in most patients, whether having failed biologic or targeted synthetic disease-modifying anti-rheumatic drug (b/tsDMARD) therapies or b/tsDMARD-naïve, is truly exciting for patients living with this disease and the field of RA treatment,” said Jonathan Graf, M.D., professor of Medicine, Division of Rheumatology at the University of California, San Francisco, and a lead investigator for the trial.
The U.S. RA market is valued at more than $20 billion. Even after patients have progressed on first-line biologics (anti-TNFs), the second line+ RA market generates more than $10 billion in annual revenue. For instance, rituximab, a B cell depleter with a significant adverse event profile including an increased risk of infection, prior to going off patent in 2018 and the entrance of biosimilars, achieved nearly $2 billion in annual RA sales. And today, despite biosimilar competition, it still maintains a significant share of last-line therapy sales in RA. Since 2012, no new treatment classes have been approved, and many RA patients cycle through different treatment therapies2, with up to a quarter not finding symptom relief.3
Understanding the role of pathogenic T cells in RA
In people with RA, more than 80% of T cells found in the joint tissue or synovium are pathogenic4, and their peripheral blood contains twice as many pathogenic T cells as found in non-RA patients.5 Selectively depleting pathogenic T cells in both the synovium and peripheral blood normalizes the remaining T cell composition, including regulatory T cells (Tregs), to be more reflective of a healthy immune system, leading to reduced inflammation and a modified disease state that supports durable remission of disease.
Research shows that in the periphery, rosnilimab depletes more than 90% of the pathogenic T cells (largely peripheral helper (Tph), follicular helper (Tfh) and effector (Teff) T cells), which represent only a small portion of overall T cell composition. In the synovium or joint tissue, rosnilimab depletes ~90% of the pathogenic T cells (largely Tph cells) (See Figure 1).6
Figure 1. Synovial biopsies show ~90% reduction of pathogenic T cells in the target tissue taken at baseline and 6 weeks of the study. Immunofluorescence was performed to identify highly activated cells.
These data are different from data generated by other pathogenic T cell-targeting therapies that only partially deplete. For example, Lilly’s PD-1 agonist peresolimab and JNJ’s JNJ-67484703 only deplete approximately 60% of the pathogenic T cells in the periphery7,8 and JNJ-67484703 only depletes ~50% pathogenic T cells in the synovium (no data available from peresolimab to date).8 Additionally, Phase 2 clinical trial data suggest efficacy of peresolimab on various endpoints deteriorated significantly after three months.9
Some of the issues with only depleting approximately half of the pathogenic T cells means the remaining half could propagate, leading to rampant inflammation and cytokine secretion, leaving patients vulnerable to potential comorbidities associated with uncontrolled, chronic inflammation.
“Rosnilimab delivered durable efficacy in this Phase 2b study while maintaining the integrity of the patient’s immune system,” explained Paul Lizzul, M.D., chief medical officer at Anaptys. “Rosnilimab selectively and potently depletes pathogenic T cells while preserving healthy, immunocompetent cells, maintaining an overall balanced immune cell composition that supports immune surveillance.”
Rosnilimab’s potential best-in-disease profile in RA
Rosnilimab’s global Phase 2b trial enrolled 424 patients with moderate-to-severe RA on background conventional DMARDs (e.g., methotrexate) and evaluated three dosing regimens of rosnilimab—100 mg and 400 mg every four weeks and 600 mg every two weeks—against placebo (1:1:1:1).
In February 2025, Anaptys reported that patients on all three doses of rosnilimab achieved statistically significant reductions in mean change from baseline in the disease activity score in 28 joints (DAS-28) C-reactive protein (CRP), the study’s primary endpoint, as well as ACR20 response at Week 12 compared to placebo. While cross-trial comparisons are for illustrative purposes only, it was encouraging to observe that during the three-month placebo-controlled period, in both b/tsDMARD-naïve and -experienced patients, rosnilimab demonstrated rapid onset of ACR20 response, an accepted Phase 3 registrational endpoint, as well as a substantial decrease in objective inflammatory biomarkers such as CRP, that appeared to be comparable to the Phase 2b trial results of the JAK inhibitor upadacitinib.10,11
At baseline, patients had high disease activity with a mean Clinical Disease Activity Index (CDAI) score of 38 and median CDAI score of 36. Of the 318 rosnilimab patients in the intent to treat (ITT) population, CDAI LDA, the level signifying disease control and requiring a CDAI score of less than 10, was achieved by 69% of patients (220 responders) across all three doses of rosnilimab at Week 14, the timepoint required to be eligible to remain in the all-active treatment period on rosnilimab through Week 28. Maximal response rates for rosnilimab have not yet been observed as strict criteria at Week 14 prevented patients with meaningful improvement from continuing treatment in the trial (See Figure 2).6
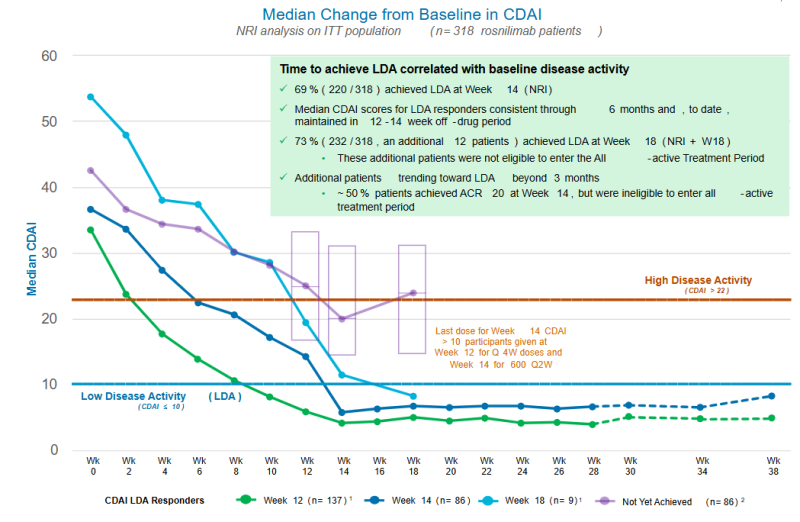
Figure 2: Green and dark blue lines reflect the 220 CDAI LDA responders who achieved LDA by Week 14 (NRI). The light blue line reflects 12 patients who achieved CDAI LDA four or six weeks after their last rosnilimab dose at their first follow-up visit (Week 18) but were ineligible to continue in the all-active treatment period and were imputed as non-responders at Week 28. The purple line reflects additional patients trending toward LDA after Week 14. On average, patients with higher disease activity take longer to achieve CDAI LDA.
At six months, rosnilimab demonstrated “JAK-like” efficacy with deepening responses on CDAI LDA, CDAI remission (CDAI ≤2.8) and ACR70. Importantly, this was also observed in only the subset of b/tsDMARD-experienced patients who received 600 mg every two weeks and 400 mg every four weeks, showing a dose response relative to the lower dose.
Patients who were in CDAI LDA at Week 28 further had durable responses off-drug for at least an additional three months, supporting the potential to test maintenance dosing with extended dosing intervals (e.g., every eight or 12 weeks) in future clinical trials.
Translational data further substantiated and corroborated rosnilimab’s clinical efficacy in this trial. A ~50% reduction in mean CRP from baseline was observed through Week 28 in patients during the all-active period. Blood and synovial biopsy biomarker data showed robust, on-target pharmacological activity, demonstrating rapid, deep, sustained reductions of pathogenic T cells and increases in total Tregs.
“Impressive translational data provide further evidence that by targeting specific pathogenic T cells, rosnilimab has a substantial impact downstream on multiple known pathways that drive RA pathogenesis, with the potential to restore immune homeostasis necessary to achieve meaningful, long-lasting disease remission,” said Dr. Graf.
Favorable safety and tolerability
Consistent with prior rosnilimab studies, a favorable safety and tolerability profile across all rosnilimab doses was observed in this Phase 2b study with very few dropouts, and no treatment-related serious adverse events (SAEs), malignancies, anaphylaxis or systemic hypersensitivity. Relative to placebo, there was a low and balanced incidence of injection site reactions and serious infections. Most adverse events were mild or moderate.
“To date, rosnilimab has shown a safe and well tolerated profile with almost all patients choosing to stay on therapy through the end of the study. Rosnilimab has not demonstrated any concerning safety trends or signals, such as those seen with most advanced therapies for RA,” said Paul Emery, M.D., Versus Arthritis professor of rheumatology at the University of Leeds and Leeds Biomedical Research Centre, UK. “This is remarkable, given these patients have a two-to-threefold increased risk of comorbidities such as cardiac events, malignancies and infections, before accounting for the impact of background DMARDs, mostly methotrexate.”
Rosnilimab has been discovered and developed by San Diego-based AnaptysBio. Learn more here.
References
- Anaptys Announces Positive Rosnilimab Data Updated Through Six Months in Phase 2b Trial in RA. News release. AnaptysBio, Inc. June 3, 2025. Accessed June 15, 2025. https://ir.anaptysbio.com/news-releases/news-release-details/anaptys-announces-positive-rosnilimab-data-updated-through-six
- Kavuncu V, Evcik D. Physiotherapy in rheumatoid arthritis. MedGenMed. 2004;6(2):3. Published 2004 May 17.
- Zhao SS, Kearsley-Fleet L, Bosworth A, Watson K; BSRBR-RA Contributors Group, Hyrich KL. Effectiveness of sequential biologic and targeted disease modifying anti-rheumatic drugs for rheumatoid arthritis. Rheumatology (Oxford). 2022;61(12):4678-4686. doi:10.1093/rheumatology/keac190
- Guo Y, Walsh AM, Canavan M, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One. 2018;13(2):e0192704. Published 2018 Feb 28. doi:10.1371/journal.pone.0192704
- Chen YJ, Chen Y, Chen P, Jia YQ, Wang H, Hong XP. Characteristics of PD-1+CD4+ T cells in peripheral blood and synovium of rheumatoid arthritis patients. Clin Transl Immunology. 2024;13(10):e70006. Published 2024 Sep 27. doi:10.1002/cti2.70006
- AnaptysBio. Rosnilimab: Updated Phase 2b Data in RA. June 3, 2025. Available at: https://ir.anaptysbio.com/static-files/6cd574c5-b4fa-42f8-873f-93e9af2de251
- Benschop R, Tuttle J, Emery P, et al. AB0502 Cellular and Proteomic Changes Following Administration of Peresolimab, in a Phase 2a Trial in Rheumatoid Arthritis. Annals of the Rheumatic Diseases 2024;83:1521.
- Ling I, Marciniak S, Clarke S, et al. POS0597 Safety, Tolerability, and Activity of JNJ-67484703 in Participants with Active Rheumatoid Arthritis: Results of a Multicenter, Double-blind, Placebo-controlled, randomized, Multiple-dose Phase 1b Study. Annals of the Rheumatic Diseases, 2025;84;794-795.
- Tuttle J, Drescher E, Simón-Campos JA, et al. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. N Engl J Med. 2023;388(20):1853-1862. doi:10.1056/NEJMoa2209856
- Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and Safety of ABT-494, a Selective JAK-1 Inhibitor, in a Phase IIb Study in Patients With Rheumatoid Arthritis and an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2016;68(12):2857-2866. doi:10.1002/art.39808
- Kremer JM, Emery P, Camp HS, et al. A Phase IIb Study of ABT-494, a Selective JAK-1 Inhibitor, in Patients With Rheumatoid Arthritis and an Inadequate Response to Anti-Tumor Necrosis Factor Therapy. Arthritis Rheumatol. 2016;68(12):2867-2877. doi:10.1002/art.39801
